The gene therapy sector is poised to deliver groundbreaking treatments for rare diseases. Gene therapy startups are emerging as key players in the field by offering innovative solutions that target genetic abnormalities. For one, Spark Therapeutics, founded in 2013, developed the first FDA-approved retinal gene therapy. In our Gene Therapy Patent Landscape Report, we looked into various startups, examined their notable patents and explored various developments in gene therapy. Here are some of the gene therapy startups that are making innovative contributions to this transformative field.
Gene Therapy Startups
Kriya Therapeutics
Kriya Therapeutics, a biopharmaceutical company specializing in gene therapies for ophthalmology, neurology, and metabolic diseases, significantly bolstered its financial resources in 2023. The company added $150 million to its Series C financing, bringing the total to $430 million. Kriya made a strategic move such as acquiring Tramontane Therapeutics Inc., a Spanish startup, to enhance its capabilities in developing a gene therapy for treating nonalcoholic steatohepatitis (NASH). Additionally, Kriya unveiled its gene therapy program for thyroid eye disease.
Generation Bio
Generation Bio is a biotechnology company developing genetic medicines for genetic disorders. Their approach includes a non-viral genetic medicine platform comprising immune-quiet DNA (iqDNA), a targeted lipid nanoparticle (ctLNP) delivery system, and a rapid enzymatic synthesis (RES) manufacturing process. The iqDNA facilitates the transfer of genes to cells, offering durable treatments for rare diseases, with the flexibility for additional dosing tailored to individual patient needs. Furthermore, their ctLNP technology enables personalized treatment by targeting specific cell types and tissues. The RES manufacturing process ensures quick production of doses and the exploration of novel DNA structures.
One of their patent applications, US20220290186, titled “Gene Editing Using a Modified Closed-Ended DNA (ceDNA),” discloses a non-viral, capsid-free DNA vector with covalently-closed ends, known as a “closed-ended DNA vector” (ceDNA vector). This ceDNA vector is designed for gene editing and addresses several limitations of existing gene therapy and gene editing methods that use adeno-associated virus (AAV) vectors.
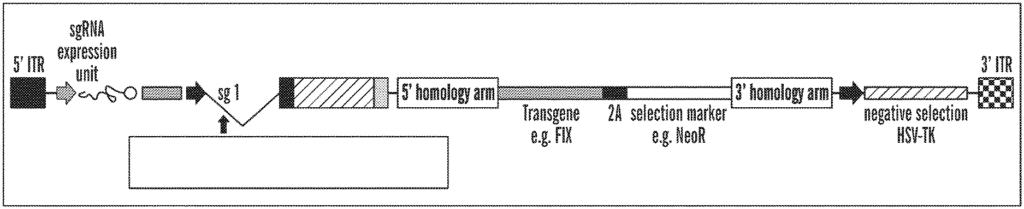
Packaging Capacity. Traditional AAV vectors have a limited viral packaging capacity (about 4.5 kb), which restricts the size of genetic material that can be delivered. The ceDNA vector overcomes this limitation with a higher capacity for transgene insertion.
Immunogenicity: AAV vectors induce immune responses that can prevent re-administration and limit treatment effectiveness. The ceDNA vector, being capsid-free, avoids triggering significant immune responses, allowing for repeated administrations if necessary.
Targeting Precision: The ceDNA vector allows for precise targeting of nuclease activity to specific DNA locations without the need for designing new proteins for each target sequence, thereby improving the efficiency and accuracy of gene editing.
Spark Therapeutics
Spark Therapeutics develops gene therapies for various diseases such as inherited retinal disease, hemophilia, lysosomal storage disorders, and neurodegenerative diseases, using adeno-associated viral (AAV) vectors. Luxturna, their AAV gene therapy, was the first FDA-approved treatment for a hereditary disease. It is also the first FDA approved retinal gene therapy, administered directly into the eye to target conditions resulting from mutations in the RPE65 gene.
In 2023, they partnered with Neurochase to develop gene therapies for rare CNS (central nervous system) diseases, combining Neurochase’s drug delivery expertise with Spark’s AAV platform. Spark also established a gene therapy innovation center at Drexel University in Philadelphia.
Their patent application (US20160346359) aims to treat ocular diseases using an adeno-associated virus (AAV) system. This system utilizes a nucleic acid encoding a CRISPR-Cas9 system for precise gene correction or disruption. The system involves employing multiple AAV vectors, each serving different functions: one encoding Cas9 or its ortholog, one containing the guide RNA sequence for targeted cleavage, and one containing the donor cDNA sequence of the mutated gene to be inserted at the cleavage site, with varying administration ratios. Alternatively, the system may use two vectors, one encoding a shorter Cas9 ortholog with the guide RNA encoded in cis, and the other containing the donor cDNA sequence. For larger genes, the donor may contain specific portions for correction. Additionally, a single AAV vector containing the CRISPR-Cas9 system may also be employed for targeted gene cleavage.
Voyager Therapeutics
Voyager Therapeutics specializes in gene therapies for neurological diseases, utilizing their TRACER™ platform to identify AAV capsids that can penetrate the blood-brain barrier effectively and shows enhanced central nervous system tropism across different species. Recently, Voyager partnered with Novartis to develop gene therapy candidates for genetic disorders like Huntington’s disease and spinal muscular atrophy.
U.S. Patent No. 11,752,181 describes adeno-associated viral (AAV) particles carrying siRNA molecules for the treatment of Huntington’s Disease (HD). It provides viral genomes with sequences targeting HTT to reduce its expression in cells or subjects. These genomes consist of various regions, including inverted terminal repeat (ITR) sequences, enhancers, promoters, introns, modulatory polynucleotides encoding siRNA, polyadenylation signals, and 3′ ITR sequences. These elements collectively facilitate the efficient delivery and expression of siRNA molecules to target HTT and alleviate HD-related symptoms.
Find out more about key gene therapy startups and their innovation in our Gene Therapy Patent Landscape Report.





