Gene therapy offers a revolutionary approach to treating genetic disorders by introducing healthy genetic material into a patient’s cells. Innovations in gene editing, particularly the advent of CRISPR technology in 2009, have significantly accelerated the development of gene therapy.
What is Gene Therapy?
Gene therapy is a groundbreaking technique aimed at modifying or manipulating the expression of genes or altering the biological properties of living cells for therapeutic purposes. This innovative approach encompasses two main methods: gene addition, which inserts healthy genes, and gene editing, which modifies DNA sequences.
By targeting the root cause of diseases, these techniques offer the potential for long-lasting and even permanent treatments. Gene therapy can be delivered directly into the body or through modified cells that are reintroduced into the patient.
Moreover, this approach extends its applications beyond genetic disorders to include cancer treatment. A notable example is CAR T-cell therapy, which enhances the patient’s own T cells to specifically target and eliminate cancerous cells.
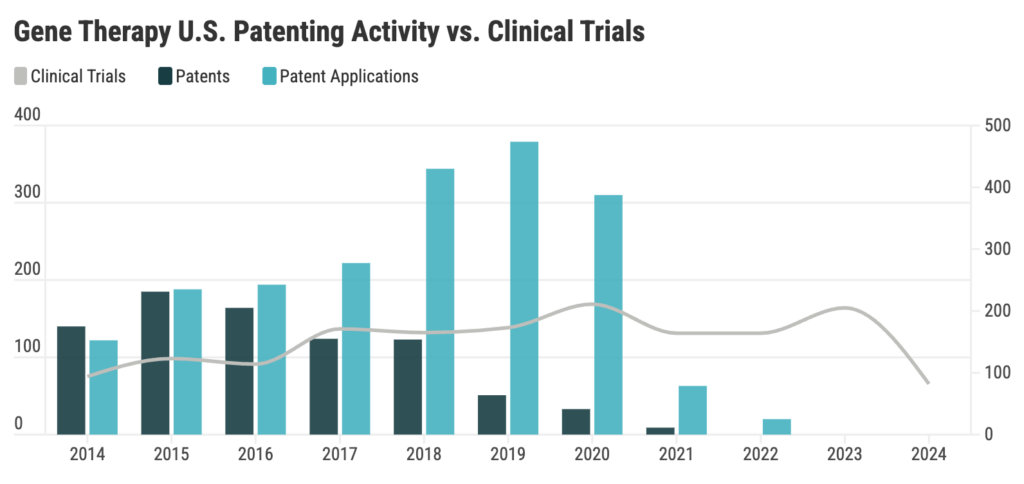
Gene therapy patent filings surged in 2018 but significantly declined by 2020
In our recent Gene Therapy patent landscape report, data shows a peak in patenting activity in 2018, following the FDA’s approval of the first gene therapy product in 2017. The momentum in gene therapy advancements continued, culminating in a record-breaking seven gene therapies approved by the US FDA in 2023, bringing the total number of FDA-approved gene therapy products to 37. However, based on our dataset, the rate of patent filings and patent grants have significantly declined.
There are many factors that may have shaped the decision to file patents. One of these factors is the question of patentability In Association for Molecular Pathology v. Myriad Genetics, Inc., a landmark ruling in 2013, it was held that isolating genes that are naturally found in the human body does not constitute an invention eligible for patent protection. Other factors include the proper timing of patent applications relative to clinical trials, FDA approvals, a more stringent regulatory oversight and more.
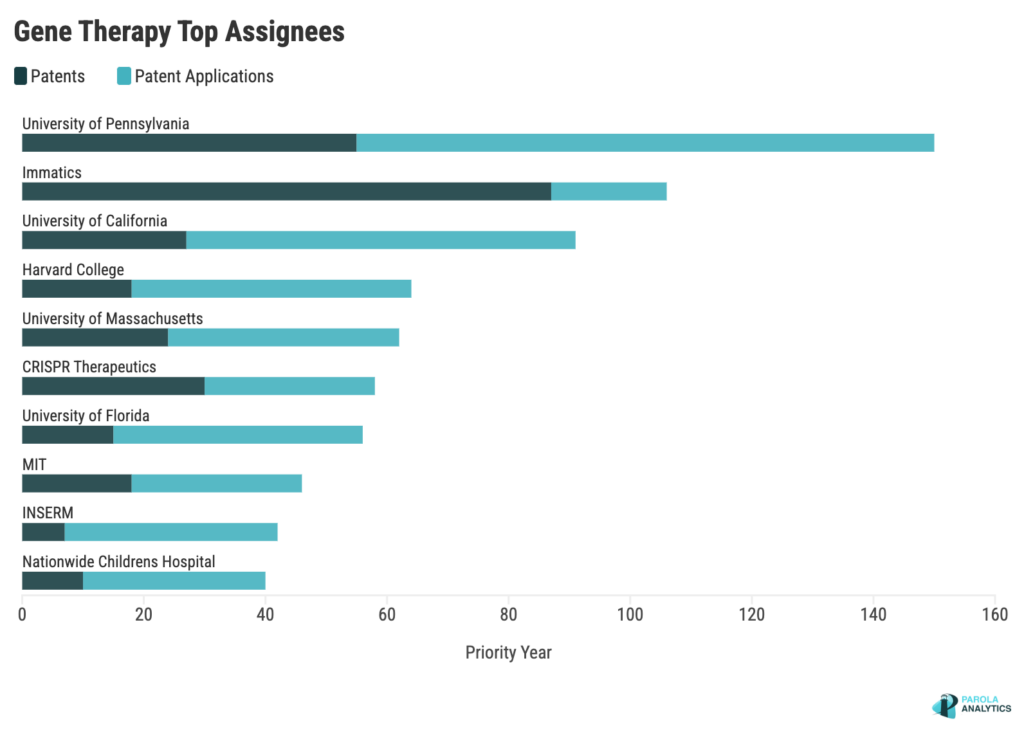
UPenn, Immatics lead in gene therapy patents
In the dataset which includes 2,671 patents and patent applications (P/PAs), about 26% are with the top 10 assignees. Renowned academic institutions like the University of Pennsylvania (UPenn) and the University of California (UC System), alongside innovative biotech companies like Immatics and CRISPR Therapeutics, offer distinct expertise and advancements, collectively propelling genetic medicine forward.
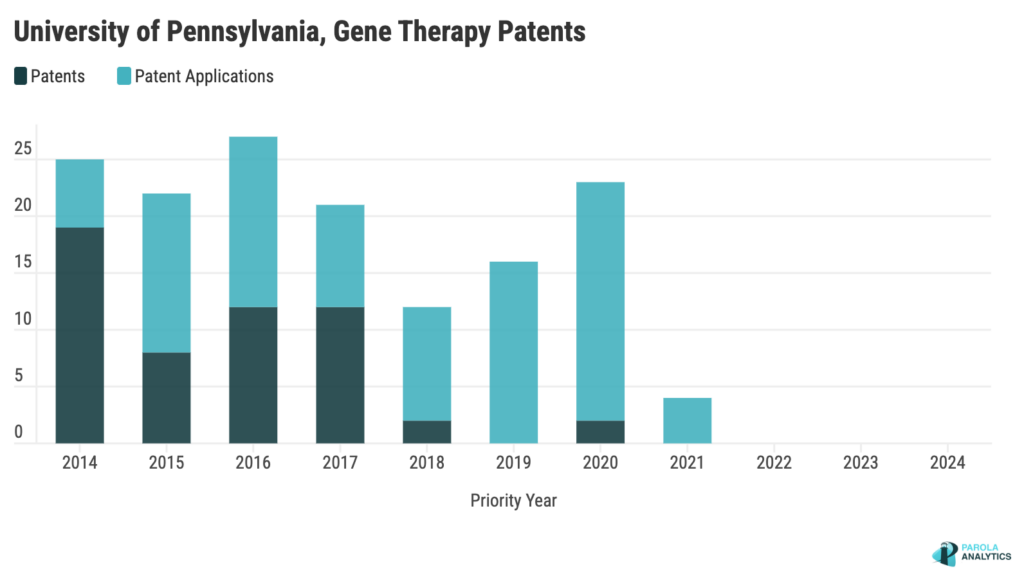
Over the last decade, UPenn’s patenting activity has been consistent, with consistent filings from 2014 to 2021. UPenn has a famous Gene Therapy Program (GTP) and has made significant contributions in refining adeno-associated viruses (AAV) as effective gene transfer vectors. The peak in 2016 coincides with the unveiling of UPenn’s AAV 3.0™, a program aimed at advancing viral vectors for gene therapies and genome editing.
UPenn’s impact extends beyond academia through collaborations with industries like Novartis and Amicus Therapeutics. The partnership with Novartis, which began in 2012 and ended in 2019, resulted in groundbreaking achievements, including the development and approval of Kymriah, the first gene therapy approved for cancer globally. Additionally, joint patents with Novartis, such as the U.S. Patent No. 9,777,061, disclose methods for treating cancer using CAR-T cell therapy targeting the CD33 antigen. In 2018, UPenn’s GTP partnered with Amicus Therapeutics to develop AAV gene therapies for various conditions, expanding in 2019 to include lysosomal disorders and additional rare diseases. Notably, a patent application (U.S. Pat. App. No. 2022/0193261) co-assigned to UPenn and Amicus discloses a recombinant AAV for treating Pompe disease, targeting muscles, heart, and motor neurons affected by the condition.
Find out more about the key innovations areas of the top assignees in our Gene Therapy Patent Landscape Report.
Key innovation areas
As of March 2024, the U.S. Food and Drug Administration (FDA) has approved several genetic therapies which include therapies for inherited eye conditions, different types of cancer, and several blood disorders.
In 2017, Luxturna became the first FDA-approved gene therapy for an inherited condition potentially causing blindness. It was initially developed by the University of Pennsylvania and Children’s Hospital of Philadelphia (CHOP). The latter stages and manufacturing were handled by Spark Therapeutics, also founded by CHOP researchers. Luxturna targets vision loss resulting from mutations in RPE65 genes which are crucial for converting light into electrical signals in the eye. Luxturna employs altered AAVs carrying functional RPE65 genes, directly repairing retinal cells to restore vision.
In our dataset, 39 patents and patent applications specifically mentioned “RPE65” in the claims and 401 P/PAs were identified for various eye disorders, as indicated by mentions of keywords such as “eye” or “retina” in the claims. Cancer treatments using gene therapy represent 35% of our dataset. Other FDA approved products are for several blood disorders namely sickle cell disease, hemophilia, and ß-thalassemia. In our dataset, there are about 126 P/PAs which mention hemophilia, 68 for sickle cell disease, and 67 for thalassemia.
Startups
Several startup biopharmaceutical companies are making significant strides in gene therapy development for various medical conditions
Kriya Therapeutics, a biopharmaceutical company, develops therapies for eye, brain, and metabolic disorders. They recently acquired Tramontane Therapeutics Inc., bolstering their efforts to develop a gene therapy for NASH. Additionally, they unveiled a gene therapy program for thyroid eye disease.
Spark Therapeutics specializes in gene therapies for various diseases, including inherited retinal disease, hemophilia, lysosomal storage disorders, and neurodegenerative diseases. They have developed a gene therapy platform utilizing adeno-associated viral (AAV) vectors, with Luxturna being the first FDA-approved AAV gene therapy for a genetic disease. Spark also partnered with Neurochase in 2023 to develop gene therapies for rare CNS diseases, integrating Neurochase’s drug delivery expertise with Spark’s AAV platform.
Gene therapy holds immense promise for the roughly 400 million individuals worldwide affected by genetic diseases. Several institutions are actively harnessing this potential to revolutionize medical care, with a focus on providing cures for genetic disorders. The field is witnessing a surge in clinical trials and approved products, with ongoing advancements in gene editing and viral vector delivery systems enhancing treatment safety and efficacy.
Find out more about the patenting activity in this space, key innovation areas and in-depth look at the notable patents in this space. Download our Gene Therapy Patent Landscape Report.