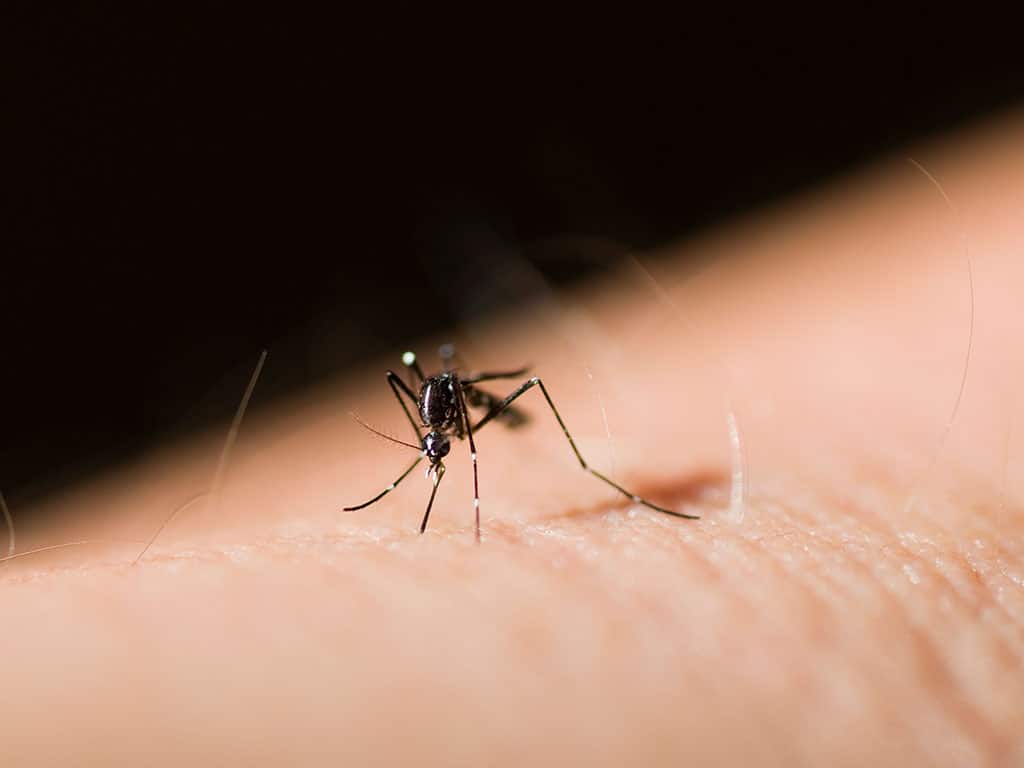
The World Health Organization has recommended the RTS,S/AS01 (RTS,S) malaria vaccine for widespread use in sub-Saharan Africa, with the intent of curbing a leading cause of childhood illness and death in the region.
The recommendation for RTS,S, also marketed as Mosquirix, was based on the advice of WHO’s global advisory bodies on immunization and malaria. The vaccine is indicated for the prevention of P. falciparum malaria in children 5 months and up living in regions with moderate to high transmission as defined by WHO. P. falciparum is the deadliest malaria parasite in the world, and the most prevalent one in Africa. The drug would be administered in 4 doses; pilot implementation studies dosed participants once for three consecutive months, with a booster shot 18 months after the first dose.
WHO touted the vaccine’s efficacy and strong safety profile, covering a significant reduction (30%) in deadly severe malaria across at least 2.3 million doses administered to date. RTS,S was also deemed cost-effective in areas of moderate to high malaria transmission.
Data on the vaccine came from ongoing pilot malaria vaccine introductions in Ghana, Kenya, and Malawi. These programs have brought RTS,S to over 800,000 children since 2019.
WHO says over 260,000 African children under the age of five die from malaria annually. “Using this vaccine on top of existing tools to prevent malaria could save tens of thousands of young lives each year,” WHO Director-General Dr. Tedros Adhanom Ghebreyesus comments.
The recommendation for RTS,S punctuates over three decades of research by pharmaceutical giant GlaxoSmithKline, along with millions in funding from The Bill & Melinda Gates Foundation. Africa has been grappling with malaria for centuries, and despite WHO’s “historic” and “groundbreaking” recommendation for the “breakthrough for science” that is RTS,S, a superior vaccine may already be just around the corner.
Earlier this year, Parola Analytics featured the patent application for another malaria vaccine, claimed to be the first one to fully immunize against the mosquito-borne disease—RTS,S, in actuality, is only about 30% effective against malaria.
The vaccine utilizes RNA technology, similar to COVID-19 immunizations from Pfizer and Moderna. It teaches the body how to create a pathogen’s unique protein in order to fight it. But while COVID-19 is fought using mRNA vaccines, the patent application describes a saRNA platform. The researchers say saRNA is more efficient, since it is effective at much lower doses because it can rapidly produce copies of itself inside the cell.
GSK would also own the supposedly superior vaccine upon its USPTO approval. Novartis Pharmaceuticals, which sold its vaccines business to GSK in 2015, funded the work along with the National Institutes of Health.