A recently published patent application covers a drug said to be more effective at treating post-traumatic stress disorder (PTSD) than current medical options, with fewer side effects to boot.
PTSD is characterized by intrusive memories, flashbacks, and nightmares, experienced following traumatic experiences. People with this chronic anxiety disorder may feel stressed or frightened even in safe environments. PTSD symptoms last for more than one month, and are severe enough to interfere with relationships or work.
Though any individual can suffer from PTSD—someone grieving a loved one or affected by a natural disaster, for instance—it is war veterans who commonly develop it. In the U.S. military, a soldier’s service era impacts the prevalence of PTSD: it is triggered in about 12% of Gulf War veterans, while almost a third of those deployed in the Vietnam War are estimated to have been chronically traumatized.
Currently, PTSD sufferers are typically prescribed antidepressants. The applicant, Aché Laboratórios, one of Brazil’s largest pharmaceutical companies, notes how only about 60% of patients respond to the existing pharmacological treatments, while only 20-30% achieve full remission. A 2011 study found that, in a large sample of the Australian population, the median time to remission is 14 years. Given the debilitating physical and psychological symptoms of extreme trauma, not to mention seven and four times the suicide risk for women and men respectively, there is a need for fast-acting and effective treatment strategies.
Aché’s invention involves using a quinazolinone compound, named IA1-29. In medicinal chemistry, quinazolinones are pharmacophoric scaffolds upon which numerous biologically active natural products, synthetic compounds, pharmaceutical drugs, agrochemicals, and veterinary products have been built.
Quinazolinone derivatives have been applied to a wide range of disorders: from malaria to diabetes to cancer. In addition, there were also quinazolinone compounds which were established as antidepressant and antipsychotic properties.
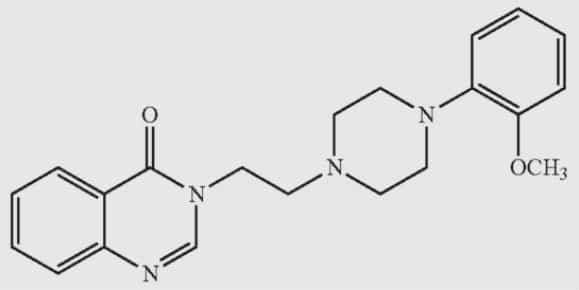
The structure of the quinazolinone compound (IA1-29) in Aché’s PTSD drug, defined as 3-(2-(4-(2-methoxyphenyl)piperazine-1-yl) ethyl) quinazoline-4(3H)-one.
IA1-29 may be orally administered as a solid like a pill or a liquid as an injection, among other examples. The company also describes an instance of the quinazolinone compound being administered in up to 5,000-milligram doses once or more times a day.
Aché’s quinazolinone compound may also be synthesized into a drug with at least another active principle, for instance chosen from antidepressant, antiretroviral, antibiotic, or anti-inflammatory, among others.
The patent filing cites a research investigation on IA1-29’s efficacy on a rodent model employing the Single-Prolonged Stress protocol (SPS). This pre-clinical approach is commonly used to test medications for PTSD. It involves a single continuous session of sequential exposure to three stressors: psychological using 2 hours of restraint, physiological through a 20-minute forced swim, and pharmacological via exposure to ether anesthesia leading to unconsciousness.
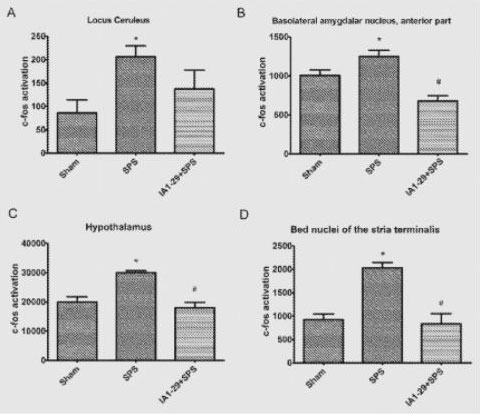
Charts corresponding to brain regions in mice not exposed to SPS, exposed to SPS, and exposed to SPS but pre-treated with IA1-29.
The test was designed to mimic the activation that occurs in the brain after a prolonged stress stimulus. Researchers measured c-fos positive neurons in four different regions of the rodent brain. The c-fos protein is an immediate early gene product that can be used as a marker of cell activation in individual neurons.
Researchers found that prior administration of IA1-29 suppressed the SPS-Induced c-fos expression in key brain regions involved in stress response. This preclinical success has the potential to produce a much-needed medical solution for PTSD, which is estimated to impact one in 11 people.
The featured patent application, “Treatment of Post-traumatic Syndrome Disorder”, was filed with the USPTO on December 14, 2018 and published thereafter on July 22, 2021. The listed applicant is Ache Laboratorios Farmaceuticos S.A. The listed inventors are Alessandra Mascarello, Cristiano Ruch Werneck Guimar Es, Hatylas Felype Zaneti De Azevedo, and Marcos Antonio Ferreira Junior.