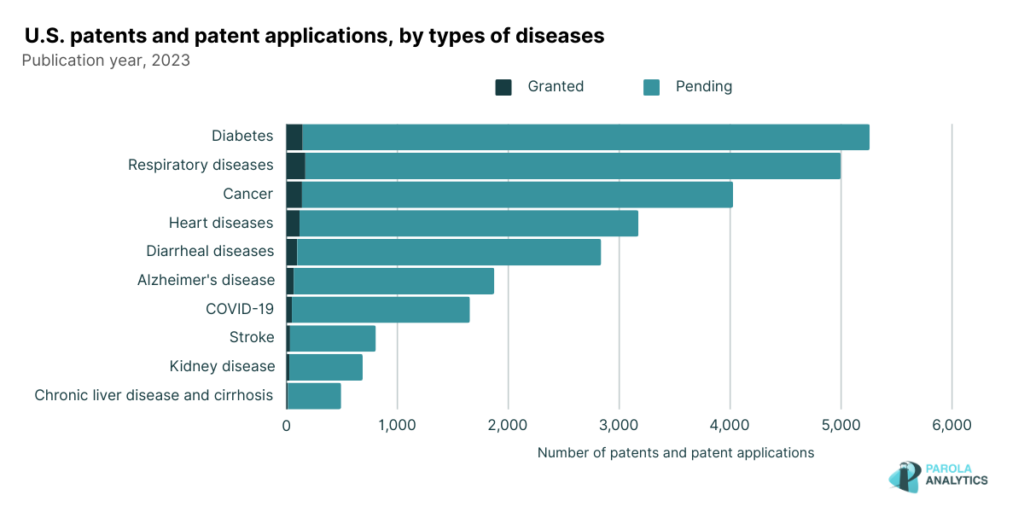
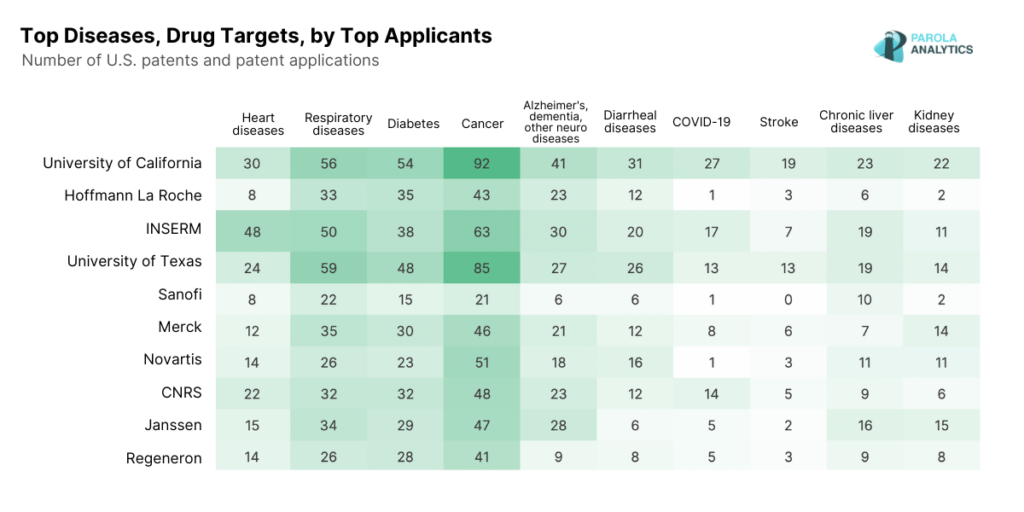
Our analysis of the U.S. pharmaceutical patent landscape in 2023 reveals that diseases like diabetes, respiratory conditions, and cancer remain at the forefront innovation. These diseases align closely with the leading causes of death reported by The US Centers for Disease Control and Prevention in 2021.
Our report on the U.S. Pharmaceuticals Patent Landscape resulted in a dataset of 21,235 U.S. patents and patent applications (PPAs) published in 2023. We put particular emphasis on the top diseases addressed, the top applicants, and key patent applications.
2023 U.S. Patent Applications, by Top Diseases and Drug Targets
Despite achievements in vaccine development, cancer treatments, and transformative drugs for conditions like obesity and rare diseases in 2023, the pharmaceutical sector still faces significant underperformance in capital markets.
A PwC outlook on the pharmaceutical industry in 2024 suggests a need for “rethinking innovation strategies”, with a particular emphasis on untapped areas like obesity and Alzheimer’s. Despite being high-risk, the market is less crowded, but has large patient populations.
In our dataset, there are 1,874 patent applications that address Alzheimer’s disease.
The recent popularity of GLP-1 drugs, specifically glucagon-like peptide-1 (GLP-1) receptor agonists like Ozempic, Wegovy, and Rybelsus is indeed an attractive investment. These three brands are the only FDA-approved semaglutide products in the market and all are owned by Novo Nordisk.
GLP-1 is a hormone that boosts insulin production, lowering blood glucose. GLP-1 receptors on cell surfaces, when activated, increase insulin secretion, inhibit glucagon release, slow gastric emptying, and enhance feelings of satiety. A GLP-1 receptor agonist is a synthetic drug, designed to mimic GLP-1 and activate its receptors to regulate blood sugar.
Our data on U.S. pharmaceuticals patents reveal that there are 81 patent applications that disclose “GLP-1 receptor agonist” and 7 patent applications disclosing “semaglutide”.
GLP-1 as potential treatment for Alzheimer’s
In a recent study published in Cell Metabolism, In a December 2023 study published in Cell Metabolism, researchers found that GLP-1 receptor agonist drugs, such as Ozempic, have been found to subdue brain inflammation, potentially offering a new treatment avenue for Alzheimer’s and Parkinson’s diseases. One of Novo Nordisk’s patent applications discloses administering semaglutide as a potential treatment for dementia.
Indeed, obesity and weight loss management are potentially promising areas for growth. are On February 5, 2024, Novo Nordisk announced that it is buying New Jersey-based drugmaker, Catalent for $16.5 billion. The acquisition is aimed to expand production of the Novo Nordisk’s obesity drug, Wegovy.
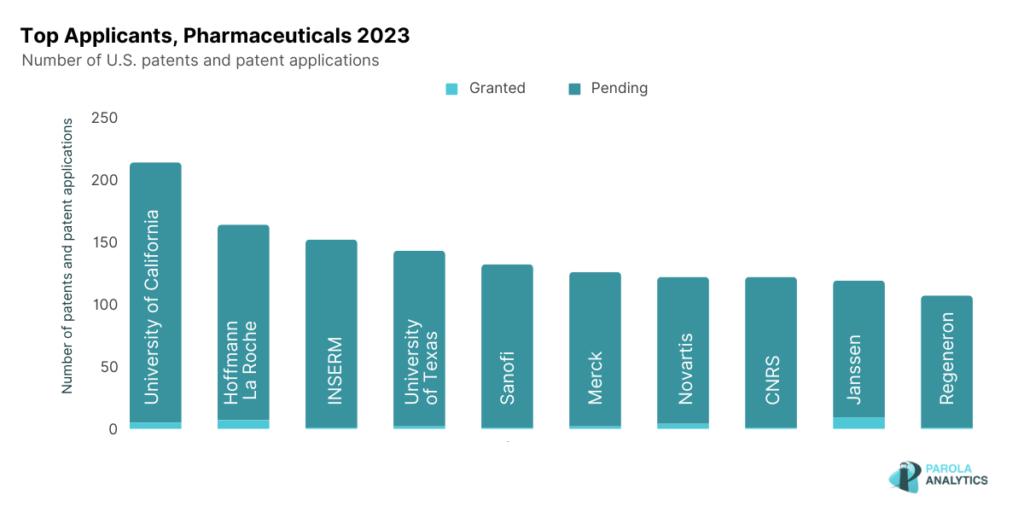
U.S. Pharmaceuticals, Top Patent Applicants 2023
The top 10 applicants in the U.S. pharmaceutical industry include universities, major pharmaceutical companies, and government research institutions. Our patent landscape report looks into their innovations in 2023, as well as the top diseases and drug targets disclosed in their 2023 patent applications.
University of California
The University of California stands out as the leader, recognized for its contributions in translating academic research into intellectual property assets. One of it’s published patent application in 2023 is titled “Nano-enabled immunotherapy in cancer.” The patent application introduces a platform technology addressing the challenge of low response rates in cancer immunotherapy, particularly for breast cancer and pancreatic ductal adenocarcinoma. The technology involves utilizing nanocarriers to deliver an indoleamine 2,3-dioxygenase (IDO) inhibitor along with an inducer of cell death (ICD) to create an immunogenic environment at the tumor site.
The nanocarriers facilitate the systemic delivery of both the IDO inhibitor and ICD-inducing agents to orthotopic pancreatic cancer sites which aims to increase the number of responders in cancer treatment.
Hoffmann-La Roche
Hoffmann-La Roche is known for innovations like Tamiflu and targeted therapies for cancer. Their patent application titled “Novel combination treatment for acute myeloid leukemia (AML)“, discloses a new drug which combines a substance blocking MDM2-p53 interaction with cytarabine for treating cancer, specifically acute myeloid leukemia, either together or sequentially.
The combination therapy consists of a first component, an inhibitor of the MDM2-p53 interaction (MDM2 inhibitor), and a second component, cytarabine. P53 is a tumor suppressor crucial for preventing cancer development, and MDM2 regulates its activity.
Dysregulation of the MDM2-p53 ratio is common in cancers, making it an attractive target for therapeutic intervention. The disclosed combination demonstrates a synergistic effect, surpassing the additive impact of individual components. The MDM2 inhibitors induce apoptosis in AML cells, and when combined with cytarabine, the therapeutic effect is heightened.
INSERM
INSERM is the French National Institute of Health and Medical Research. In November 2023, they announced a breakthrough in the treatment of Parkinson’s disease by the development of a “neuroprosthesis”. Unlike conventional methods focusing on affected brain regions, this neuroprosthesis targets the spinal cord region responsible for leg muscle activation during walking.
Their patent application, Masitinib for the treatment of sickle cell disease involves the use of a 2-aminoarylthiazole derivative, specifically masitinib or its pharmaceutically acceptable salt or solvate, for the treatment of sickle cell disease (SCD). SCD, characterized by the presence of hemoglobin variant HbS, leads to complications like vaso-occlusive crises and acute chest syndrome (ACS).
Masitinib has demonstrated efficacy in preventing these complications in a mouse model of SCD, suggesting its potential as a treatment, particularly for SCD patients at an increased risk of developing ACS due to factors like asthma or pulmonary hypertension.
Find out more about recent innovations by the top players, the top diseases and drug targets disclosed by the top applicants in our 2023 U.S. Pharmaceuticals Patent Landscape Report.