Gene therapy is revolutionizing cancer treatment, offering innovative approaches to target and eradicate cancer cells at the genetic level.
As of June 2024, the U.S. Food and Drug Administration (FDA) has approved several groundbreaking gene therapies, including those for various cancers, inherited eye conditions, and several blood disorders. Notably, eight of these FDA-approved gene therapies target different types of cancer, such as myeloma, lymphoma, leukemia, bladder cancer, and melanoma.
Our Gene Therapy Patent Landscape Report reveals that 35.3% of patents and patent applications mention cancer and related terms in their title, abstract, and claims. This highlights the significant focus on developing innovative gene therapies for cancer treatment.
Gene therapy for cancer: The Patent Landscape
Over the past decade, patenting activity for cancer gene therapies has surged, peaking in 2018. This rise came a year after the FDA’s approval of two landmark lymphoma gene therapy products: Kymriah (tisagenlecleucel), the first US FDA-approved gene therapy for any indication, used to treat acute lymphoblastic leukemia (ALL), and Yescarta, a cell-based gene therapy for adult patients with certain types of large B-cell lymphoma. Notably, most of these patents focus on innovative cancer immunotherapy treatments, reflecting a significant trend in the development of advanced cancer therapies.
FDA-approved cancer gene therapies
Kite Pharma, a biotech company, has two FDA-approved gene therapy products for cancer. In their patent application, U.S. Pat. App. No. 2022/0169694, they disclose a manufacturing method for an immunotherapy product. The method involves preparing T cells from lymphocytes and enhancing the percentages of T cells and IFN gamma-producing cells through several steps. The final immunotherapy product is then administered to cancer patients. The patent application also discloses that the T-cells may be brexucabtagene autoleucel or axicabtagene ciloleucel, among others, which are also the active ingredients in Tecartus and Yescarta, respectively.
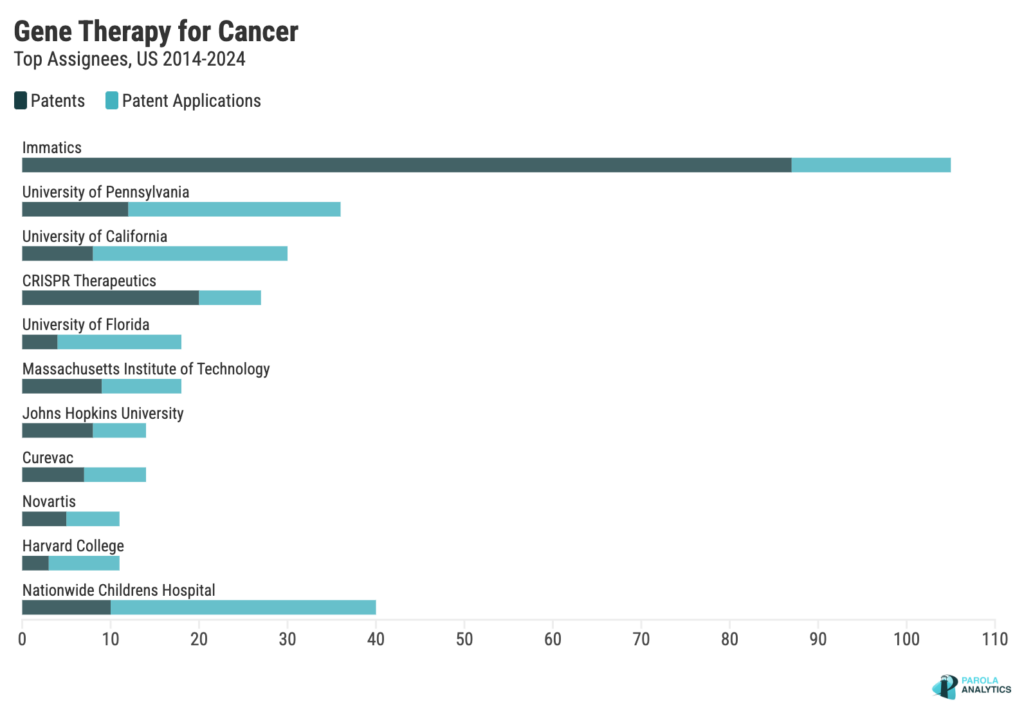
Who has the most patents in gene therapy for cancer?
Despite having no FDA approved cancer gene therapies, Immatics leads in the number of patents in the cancer gene therapy space.
We also categorized the patents and patent applications (P/PAs) related to cancer gene therapy according to common cancer types. The predominant cancer types with the highest number of P/PAs include leukemia, lymphoma, and skin cancers. This categorization aligns with the types of diseases for which FDA-approved gene therapy products are also available. Find out more in our gene therapy report.
Our report also analyzed the co-occurrence of different cancer types, it’s notable that gene therapy treatments developed for lymphoma often extend to applications in leukemia as well. Similarly, treatments initially targeted at lung cancer are also showing promise in addressing breast cancer, skin cancer, and colon and rectal cancer.
For more details, download our gene therapy patent landscape report.