Biomarkers for Down syndrome-associated conditions can be identified from blood samples, according to a recently published patent application that also details how such biomarkers can be used to develop specific treatments for affected individuals.
The discovery was submitted by the Global Down Syndrome Foundation, based in Denver. The public non-profit mentions how Down syndrome is the least funded genetic condition in the U.S. while explaining its focus on research and medical care in the area.
Down syndrome is the most common chromosomal disorder, occurring in about 1 in every 700 babies born. Trisomy 21 accounts for 95% of cases. It is when a person has an extra copy of chromosome 21, which includes genes that provide instructions for making proteins. An error in the division of egg or sperm cells during conception leads to Down syndrome.
The condition is physically characterized by low muscle tone, small stature, an upward slant to the eyes, and a single deep crease across the center of the palm. However, these traits may be present in affected individuals to varying degrees, if at all.
But the effects of Down syndrome go beyond physical traits. It is also associated with a higher incidence of cognitive impairment, congenital heart defects, and autoimmune disorders, among many others. The patent application concerns a method of evaluating a condition or disease prevalent in an individual with Down syndrome compared to a typical individual.
Comparisons are done via one or more biomarkers found in a person’s biological sample. Specifically, biomarkers are identified from a proteome analysis of blood samples. Proteomes are the sets of proteins produced across an organism, varying from cell to cell and over time. The patent says a proteome of over 3700 proteins can be assessed from plasma, the liquid portion of blood.
The document highlights the systemic proteome, which was found to be significantly altered under trisomy 21. The Discovery Study explained in the application involved blood samples from 121 individuals with Down syndrome and 54 control subjects. Two similar, subsequent validation studies demonstrated the reproducibility of changes in the systemic proteome caused by trisomy 21.
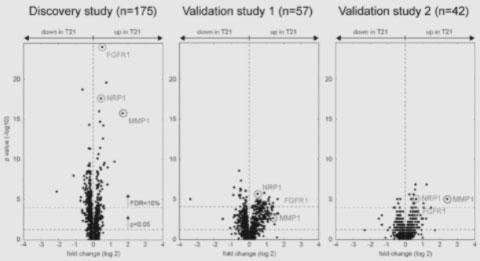
Graphs showing the p-value of proteins that were upregulated or downregulated in individuals with Down syndrome compared to typical individuals in the Discovery study, Validation study 1, and Validation study 2.
Blood tests measure for at least one biomarker indicative of the type and/or the severity of a Down syndrome-associated condition or disease. Reference values are used to confirm or refute diagnoses.
Additionally, the paper outlines a way of using the biomarker system to test the efficacy of therapeutic interventions for individuals with Down syndrome. It involves comparing biomarker levels from biological samples collected before and after a certain treatment is administered. Changes in the level of at least one biomarker are taken as an indication of a therapy’s efficacy.
The patent application lists biomarkers for Down syndrome-associated Alzheimer’s, cognitive impairment, epilepsy, leukemia, diabetes, autism, congenital heart defects, celiac disease, thyroid dysfunction, autoimmune disorders, vision problems, hearing problems, intestinal atresia, and/or sleep apnea. Measurements are also provided for heart disease, cancer, stroke, coronary artery disease, atherosclerosis, hypertension, and/or angiopathies, which are prevalent among typical individuals.
The featured patent application, “Down Syndrome Biomarkers and Uses Thereof”, was filed with the USPTO on January 19, 2017 and published thereafter on July 8, 2021. The listed applicant is the Global Down Syndrome Foundation. The listed inventor is Thomas Blumenthal.