Ford seeks patent for clear N95 masks that allow human expression
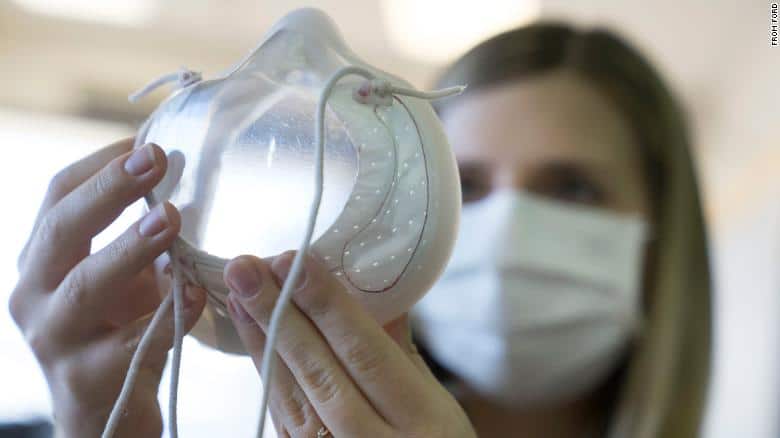
Of all the negatives brought about by the COVID-19 pandemic, perhaps the most understated is its impact on human communication. Even in cases where some establish close contact amid quarantine measures, mandates to wear face masks manage to hamper people’s ability to express themselves.
Drug patent listing and exclusivity clarified in new US bill
The Orange Book Transparency Act of 2020 (H.R. 1503) was signed into law last January 5, 2021. The Act updates regulations of the US Food and Drug Administration concerning the Approved Drug Products with Therapeutic Equivalence Evaluations (more commonly known as the Orange Book) which contains publicly available patent and exclusivity information, particularly on approved and available generic drugs.
US denied dismissal of contract breach claims in Gilead patent dispute

An interesting corner of the growing world of AI is its adoption in the automotive industry. As automakers are racing to develop driverless vehicles for widespread adoption, they recognize that leveraging AI-powered solutions and features will be the key differentiators for their brand.
AstraZeneca and Oxford’s COVID-19 Vaccine: Balancing Profit Motive and Public Interest

Just weeks after Pfizer and Moderna unveil successful clinical trials for their COVID-19 vaccine candidates, a third pharmaceutical giant makes headway with its potential answer to the pandemic. On November 23, Britain’s AstraZeneca published interim trial results for AZD1222, a vaccine it co-developed with the University of Oxford.
Pfizer/BioNTech COVID-19 vaccine gets approval for UK rollout

UK regulator Medicines and Healthcare Products Regulatory Authority (MHRA) has given emergency approval to Pfizer and BioNTech’s vaccine on December 2, making the UK the first country to approve a COVID-19 vaccine for widespread use.
A Tale of Two Vaccines: A Closer Look at Pfizer and Moderna

On November 16, Moderna claimed the latest breakthrough with its vaccine candidate mRNA-1237, deemed 94.5% effective against COVID-19 — just within striking distance of Pfizer’s vaccine, which demonstrated 95% efficacy according to the pharma giant.
