As the COVID-19 pandemic spreads worldwide and the number of affected individuals continues to grow, the race is on to find a cure for the novel coronavirus. COVID-19 is a disease caused by SARS-CoV-2, a strain from the coronavirus family that causes illness from as mild as the common cold to more severe cases such as pneumonia, severe acute respiratory syndrome, kidney failure, and to the most extreme case, death.1
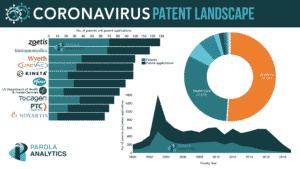
Parola Analytics recently did a report on the Coronavirus patent landscape for the past 20 years. The report includes top players coming from pharmaceutical companies, some of which are actively working on the COVID-19 research and have previously developed treatments or diagnosis to previously identified coronaviruses.
Here are some of the companies currently involved in the COVID-19 research and their corresponding patenting summary.
CureVac
CureVac is a German company specializing in messenger RNA (mRNA) medicines for cancers and rare diseases. Recently, the European Commision provided an €80M aid to support R&D and production of COVID-19 coronavirus vaccine.2 The aid is expected to be able to fund for CureVac’s 4th manufacturing site and is expected to produce around 1 billion vaccine doses once CureVac’s mRNA vaccine candidate is validated.3
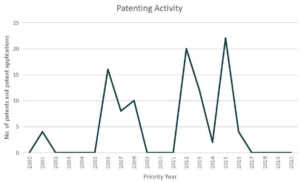
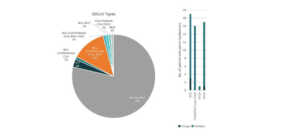
Based on our analysis, CureVac ranks fourth in the coronavirus patent landscape.
Patents and patent applications: 98
P/PA: 98
| Sample Patent |
|---|
| WO2009127230A1 |
| Modified (m)RNA for Suppressing of Avoiding an Immunostimulatory Response and Immunosuppressive Composition |
| The present invention relates to a modified (m)RNA suitable for suppressing and/or avoiding an innate immunostimulatory response in a mammal typically exhibited when administering the corresponding unmodified (m)RNA and an immunosuppressive composition comprising this RNA. The invention furthermore relates to a pharmaceutical composition containing said modified (m)RNA. The invention also relates to the use of said modified (m)RNA or immunosuppressive composition (for the preparation of a medicament, e.g. a pharmaceutical composition) and/or the use of the pharmaceutical composition for suppressing and/or avoiding an immune response in a mammal when administering said pharmaceutical composition for the treatment of various diseases. Finally, the invention relates to kits containing the immunosuppressive composition and/or the pharmaceutical composition. |
| No. of Forward Citations: 228 |
Pfizer
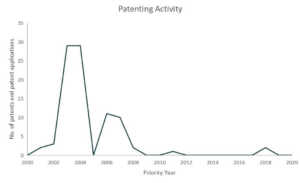
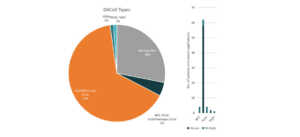
Since 2018, Pfizer has been in collaboration with immunotherapy company, BioNTech, for the development of mRNA-based vaccines for influenza and in March 17, both companies announced that they will also co-develop a potential mRNA-based vaccine for preventing COVID-19 infection.4 Pfizer also issued a five-point plan to tackle COVID-19. The plan includes call for the biopharmaceutical industries to share tools and insights that lead or will lead to breakthroughs. A SWAT team composed of Pfizer’s experts was also created which sole purpose is to produce a plan to address the COVID-19 pandemic. Pfizer has also offered to share their clinical development and regulatory expertise as well as their excess manufacturing capacity in the event that a therapy or vaccine is already approved.5 Pfizer also belongs to the top players in the coronavirus patent landscape.
Patents and patent applications: 89
| Sample Patent |
|---|
| WO200793901 |
| 3 -deazapurine derivatives as tlr7 modulators |
| The present invention relates to immune response modifiers of Formula (I), which act selectively through agonism, of Toll-Like Receptors (TLRs), uses thereof, processes for the preparation thereof, intermediates used in the preparation thereof and compositions containing said inhibitors. These inhibitors have utility in a variety of therapeutic areas including the treatment of infectious disease such as Hepatitis (e.g. HCV, HBV), genetically related viral infection and cancer. |
| No. of Forward Citations: 158 |
Regeneron Pharmaceuticals
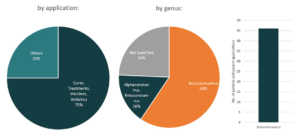
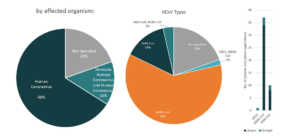
Initially used as a treatment for rheumatoid arthritis, Kevzara, is now a potential candidate for COVID-19 treatment. Kevzara, which is developed through the partnership of Sanofi Pasteur and Regeneron, will be employed at a clinical trial in New York, one of the epicenters of the pandemic in the US.6 Kevzara is one of the three-front plans of Regeneron to battle COVID-19. Another is a plan to create a vaccine which they estimate would take up to at most 2 years to account for safety testing. Lastly, their third approach is to create a new treatment. They are going to repurpose their Yancopoulus technique for Ebola and try it for COVID-19. Regeneron will start testing of this technique in June.7 Aside from Kevzara, Regeneron has also identified hundreds of antibodies for coronavirus treatment and that they will select the top antibodies to create a treatment for large-scale manufacturing by April.8 Based also on the Patents and patent applications portfolio of Regeneron, they seem to be focusing on human betacoronaviruses especially for MERS-CoV.
Patents and patent applications: 56
| Sample Patent |
|---|
| US9718872 |
| Human antibodies to middle east respiratory syndrome—coronavirus spike protein |
| The present invention provides monoclonal antibodies that bind to the Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) spike protein, and methods of use. In various embodiments of the invention, the antibodies are fully human antibodies that bind to MERS-CoV spike protein. In some embodiments, the antibodies of the invention are useful for inhibiting or neutralizing MERS-CoV activity, thus providing a means of treating or preventing MERS infection in humans. In some embodiments, the invention provides for a combination of one or more antibodies that bind to the MERS-CoV spike protein for use in treating MERS infection. In certain embodiments, the one or more antibodies bind to distinct non-competing epitopes comprised in the receptor binding domain of the MERS-CoV spike protein. |
| No. of Forward Citations: 18 |
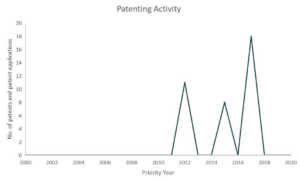
Gilead
In partnership with several US government health agencies, Gilead is currently exploring Remdesivir’s potential as treatment for COVID-19. Currently, Remdesivir is still an experimental medicine and is still in cilinical studies to evaluate its safety and efficacy.9 Last March 22, Gilead temporarily limited access to Remdesivir due to an overwhelming demand and restricted the medicine supplies to COVID-19 patients that are pregnant and children under 18 years old that shows severe symptoms.10
Patents and patent applications: 37
| Sample Patent |
|---|
| WO201749060 |
| Methods for treating arenaviridae and coronaviridae virus infections |
| Provided are methods for treating Arenaviridae and Coronaviridae virus infections by administering nucleosides and prodrugs thereof, of Formula (I): wherein the 1' position of the nucleoside sugar is substituted. The compounds, compositions, and methods provided are particularly useful for the treatment of Lassa virus and Junin virus infections. |
| No. of Forward Citations: 4 |
Sanofi Pasteur
Sanofi Pasteur is Sanofi’s vaccines global business unit; they are hoping that their previous work with SARS-CoV vaccine may give them leverage and produce a COVID-19 vaccine faster. Aside from its partnership with Regeneron Pharmaceuticals, the company is also working with the Biomedical Advanced Research and Development Authority (BARDA) and will use its recombinant DNA platform for COVID-19.11
Patents and patent applications: 14
| Sample Patent |
|---|
| JP5138601 |
| Recombinant virus stabilized formulation |
| This invention relates to preparations of viruses, e.g. for vaccine or other pharmaceutical or research use, to their stabilization, and to processes of producing such preparations, as well as to their use, e.g. as vaccines or as virus vectors. The formulations comprise a sugar, a preservative, a dispersing agent, a thermal stability agent, a buffer, and up to three distinct types of amino acids without impacting the structural appearance of the lyophilized product. |
| No .of Forward Citations: 8 |
Moderna Therapeutics
Founded in 2010, Moderna is a company that offers to create a new generation of transformative medicines using mRNA science. Currently, Moderna is working on a potential vaccine for COVID-19 through their infectious diseases research team. Two days after the Chinese authorities released the genetic sequence of the novel coronavirus, the US NIH and Moderna also finalized the sequence for mRNA-1273, their potential vaccine. The company then proceeded to start the Phase 1 of clinical trials for mRNA-1273 as vaccine for COVID-19. Last March 16, the first participant for the trial was dosed with mRNA-1273.12
Patents and patent applications: 5
| Sample Patent |
|---|
| WO2018170347A1 |
| Zoonitic disease RNA vaccines |
| The disclosure relates to Lassa virus, Nipah virus, and betacoronavirus ribonucleic acid vaccines as well as methods of using the vaccines and compositions comprising the vaccines. |
| No. of Forward Citations: 10 |
BioFire
BioFire produces panel test for viruses, bacteria, parasites, yeast, and antimicrobial resistance genes. Currently, they are also producing COVID-19 test kits and a respiratory panel. The BioFire® COVID-19 test is fully automated, sample-to-result assay for detection and expected to deliver results within an hour while the BioFIre® Respiratory 2.1 Panel, aside from SARS-CoV-2, can also detect 21 additional respiratory pathogens.13
Patents and patent applications: 1
| Sample Patent |
|---|
| US20180216164 |
| High density self-contained biological analysis |
| Devices, containers, and methods are provided for performing biological analysis in a closed environment. Illustrative biological analyses include high density nucleic acid amplification and detection and immune-PCR. |
| Priority Year: 2006 |
Check out our CORONAVIRUS Landscape Report
References
-
- World Health Organization, “Coronavirus,” [Online]. Available: https://www.who.int/health-topics/coronavirus. [Accessed 25 March 2020].
- H. Balfour, “EC offers €80m to CureVac for COVID-19 vaccine development,” European Pharmaceutical Review, 19 March 2020. [Online]. Available: https://www.europeanpharmaceuticalreview.com/news/115517/ec-offers-e80m-to-curevac-for-covid-19-vaccine-development/. [Accessed 24 March 2020].
- D. Stanton, “One billion doses: CureVac gains $88m to support capacity for COVID-19 mRNA vaccine,” BioProcess International, 18 March 2020. [Online]. Available: https://bioprocessintl.com/bioprocess-insider/global-markets/one-billion-doses-curevac-gains-88m-to-support-capacity-for-covid-19-mrna-vaccine/. [Accessed 25 March 2020].
- Pfizer, “Pfizer and BioNTech to Co-Develop Potential COVID-19 Vaccine,” 17 March 2020. [Online]. Available: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_and_biontech_to_co_develop_potential_covid_19_vaccine. [Accessed 24 March 2020].
- Pfizer, “Industry News: Pfizer issues five-point plan to tackle COVID-19,” SelectScience, 20 March 2020. [Online]. Available: https://www.selectscience.net/industry-news/pfizer-issues-five-point-plan-to-tackle-covid-19/?artID=50909. [Accessed 25 March 2020].
- Sanofi, “Sanofi and Regeneron begin global Kevzara® (sarilumab) clinical trial program in patients with severe COVID-19,” PRNewswire, 16 March 2020. [Online]. Available: https://www.news.sanofi.us/2020-03-16-Sanofi-and-Regeneron-begin-global-Kevzara-R-sarilumab-clinical-trial-program-in-patients-with-severe-COVID-19. [Accessed 24 March 2020].
- A. Murray and D. Meyer, “Regeneron will start testing its Covid-19 treatment in June,” Fortune, 23 March 2020. [Online]. Available: https://fortune.com/2020/03/23/regeneron-testing-coronavirus-covid-19-treatment-in-june-ceo-daily/. [Accessed 24 March 2020].
- Regeneron, “Regeneron says has identified antibodies to treat coronavirus,” Reuters, 17 March 2020. [Online]. Available: https://www.reuters.com/article/health-coronavirus-regeneron-pharms/regeneron-says-has-identified-antibodies-to-treat-coronavirus-idUSL4N2BA3FG. [Accessed 25 March 2020].
- Gilead, “Gilead Sciences Update On The Company’s Ongoing Response To COVID-19,” Gilead, [Online]. Available: https://www.gilead.com/purpose/advancing-global-health/covid-19. [Accessed 24 March 2020].
- M. Herper, “Gilead pauses access to experimental Covid-19 drug due to ‘overwhelming demand’,” Stat News, 22 March 2020. [Online]. Available: https://www.statnews.com/2020/03/22/gilead-suspends-access-to-experimental-covid-19-drug-remdesivir/. [Accessed 24 March 2020].
- Sanofi, “Sanofi joins forces with U.S. Department of Health and Human Services to advance a novel coronavirus vaccine,” PRNewswire, 18 February 2020. [Online]. Available: https://www.news.sanofi.us/2020-02-18-Sanofi-joins-forces-with-U-S-Department-of-Health-and-Human-Services-to-advance-a-novel-coronavirus-vaccine. [Accessed 24 March 2020].
- Moderna, “Moderna’s Work on a Potential Vaccine Against COVID-19,” 16 March 2020. [Online]. Available: https://www.modernatx.com/modernas-work-potential-vaccine-against-covid-19. [Accessed 19 March 2020].
- BioFire, “BioFire COVID-19 Solutions,” [Online]. Available: https://www.biofiredx.com/covid-19/. [Accessed 24 March 2020].